Μπαταρία ...λεμονιού.
Δημοσιεύτηκε: Κυρ Ιαν 18, 2009 11:30 pm
Μπαταρία ...Λεμονιού!*
Το βρήκα εξαιρετικά ενδιαφέρον και σας το μεταφέρω ακριβώς όπως αναφέρεται εδώ
(Πηγή: http://www.hilaroad.com
)
*Δεν ξέρω τι γίνεται με τα δικά μας νομίσματα.

Creating a battery from a lemon is a common project in many science text books. Successfully creating one of these devices is not easy.
Batteries consist of two different metals suspended in an acidic solution. Copper and Zinc work well as the metals and the citric acid content of a lemon will provide the acidic solution.
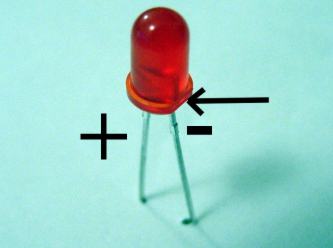
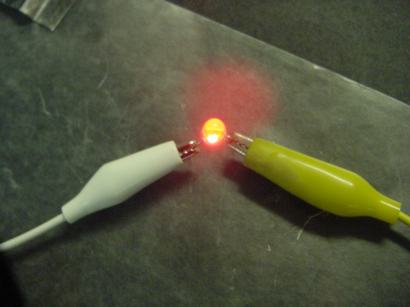
Batteries like this will not be able to run a motor or energize most light bulbs. It is possible to produce a dim glow from an LED.
The picture at the top of this page shows a basic lemon battery, a lemon, copper penny and zinc coated nail.
The lemon: A large, fresh, "juicy" lemon works best.
The nail: Galvanized nails are coated in zinc. I used a 2" galvanized common nail.
The penny: Any copper coin will work. (Canadian pennies from 1960 - 2001 all worked)

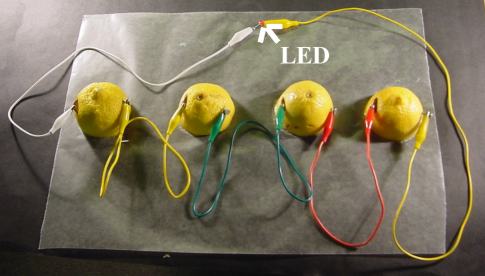
Creating the battery: Insert a penny into a cut on one side of the lemon. Push a galvanized nail into the other side of the lemon. Τhe nail and penny must not touch.
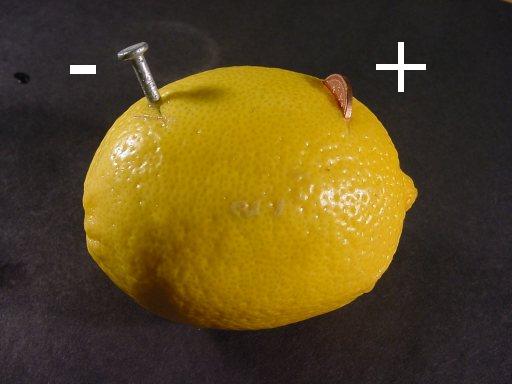
This is a single cell of a battery. The zinc nail and the copper penny are called electrodes. The lemon juice is called electrolyte.
All batteries have a "+" and "-" terminal. Electric current is a flow of atomic particles called electrons. Certain materials , called conductors, allow electrons to flow through them. Most metals (copper, iron) are good conductors of electricity. Electrons will flow from the "-" electrode of a battery, through a conductor, towards the "+" electrode of a battery. Volts (voltage) is a measure of the force moving the electrons. (High voltage is dangerous!)
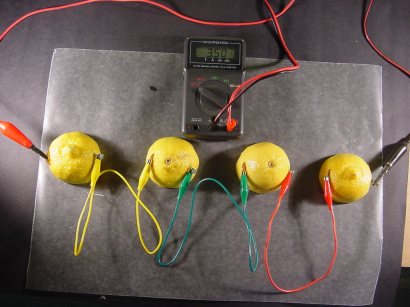
I have connected a volt meter to our single cell lemon battery. The meter tells us this lemon battery is creating a voltage of 0.906 volts.
Unfortunately this battery will not produce enough current (flowing electrons) to light a bulb.

Το βρήκα εξαιρετικά ενδιαφέρον και σας το μεταφέρω ακριβώς όπως αναφέρεται εδώ
(Πηγή: http://www.hilaroad.com
)
*Δεν ξέρω τι γίνεται με τα δικά μας νομίσματα.

Creating a battery from a lemon is a common project in many science text books. Successfully creating one of these devices is not easy.
Batteries consist of two different metals suspended in an acidic solution. Copper and Zinc work well as the metals and the citric acid content of a lemon will provide the acidic solution.
Batteries like this will not be able to run a motor or energize most light bulbs. It is possible to produce a dim glow from an LED.
The picture at the top of this page shows a basic lemon battery, a lemon, copper penny and zinc coated nail.
The lemon: A large, fresh, "juicy" lemon works best.
The nail: Galvanized nails are coated in zinc. I used a 2" galvanized common nail.
The penny: Any copper coin will work. (Canadian pennies from 1960 - 2001 all worked)

Creating the battery: Insert a penny into a cut on one side of the lemon. Push a galvanized nail into the other side of the lemon. Τhe nail and penny must not touch.

This is a single cell of a battery. The zinc nail and the copper penny are called electrodes. The lemon juice is called electrolyte.
All batteries have a "+" and "-" terminal. Electric current is a flow of atomic particles called electrons. Certain materials , called conductors, allow electrons to flow through them. Most metals (copper, iron) are good conductors of electricity. Electrons will flow from the "-" electrode of a battery, through a conductor, towards the "+" electrode of a battery. Volts (voltage) is a measure of the force moving the electrons. (High voltage is dangerous!)

I have connected a volt meter to our single cell lemon battery. The meter tells us this lemon battery is creating a voltage of 0.906 volts.
Unfortunately this battery will not produce enough current (flowing electrons) to light a bulb.